General Information
- Definition: persistent airflow limitation that is usually progressive and is associated with a chronic enhanced inflammatory response in the airways and lungs to noxious particles and gases
- due to airway and/or alveolar abnormalities
- Common, preventable & treatable disease
- Usually caused by significant exposure to noxious particles or gases
- Developed countries — smoking; developing countries — biomass fuel
- Influenced by host factors including abnormal lung development
- Chronic bronchitis — clinical diagnosis — chronic cough and sputum production
- epidemiological definition — chronic productive cough on most days during at least 3 months per year for 2 or more consecutive years
- periods of worsening or exacerbation precipitated by a respiratory tract infection
- in between exacerbations, residual clinical disease (unlike asthma where symptoms go away in between exacerbations) because chronic bronchitis is not a disease of airway hyperreactivity
- If clinical features of chronic bronchitis with evidence of airway hyperreactivity → asthmatic bronchitis or asthma-COPD overlap syndrome
- Emphysema — pathological diagnosis — destruction of lung parenchyma and enlargement of air spaces distal to the terminal bronchiole — this is the region from respiratory bronchioles to the alveoli
- where the destruction is along this path determines the subtype of emphysema
- Chronic bronchitis and emphysema can (and often do) co-exist in a patient therefore the broader term of COPD is used
- Significant comorbidities may have an impact on morbidity and mortality
Associated Anatomy & Physiology
Associated Histology
Epidemiology
Burden of COPD
COPD is underdiagnosed and under-reported
- Prevalence estimates vary widely
- Global -- 10.1%
- Deaths -- 3.2 million annually
- Third leading cause of death worldwide
- Prevalence expected to rise over next 4 decades
- Prevalence increasing in women
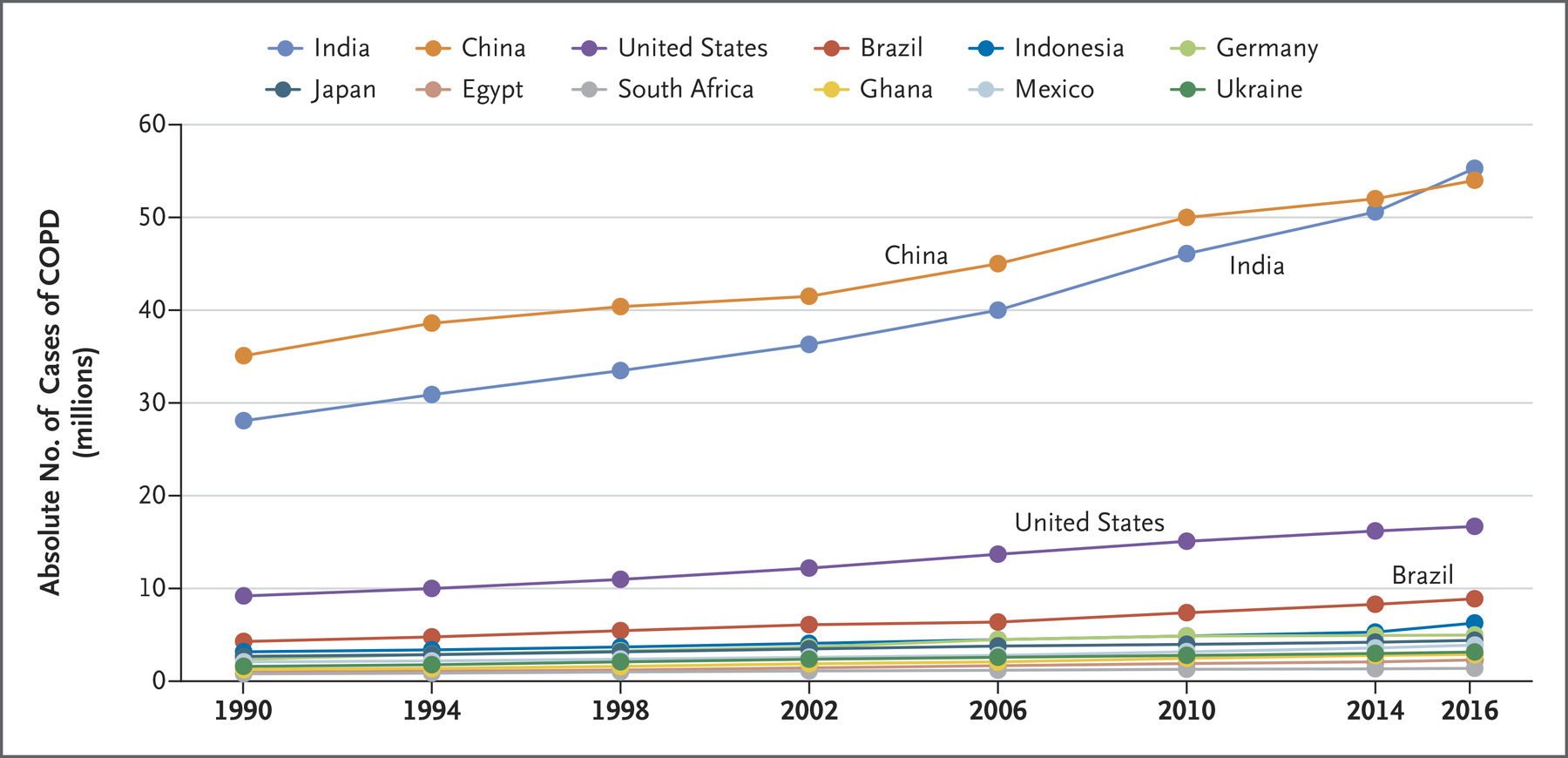
- China and India account for more than 50% of all cases of COPD in the world
- Undiagnosed COPD
- Percentage of undiagnosed COPD in the general population, aged 40 or older with current or past exposure to tobacco (≥10 pack-years) is 70-78%
- Overall health system burden by undiagnosed COPD still exists and its largely unrecognized
- Asymptomatic Undiagnosed COPD
- If FEV₁ <80% (moderate or more severe obstruction) → adverse effects ~ symptomatic COPD
- Increased risk of exacerbations and pneumonia
- Symptomatic Undiagnosed COPD
- increased risk of exacerbations, pneumonia and death
- i.e. it doesn't matter if they are symptomatic or not, being undiagnosed increases risk of adverse effects
Etiology, Pathogenesis & Pathology
Etiology & Risk Factors
- Early life factors and exposures
- Maternal smoking
- Respiratory infections
- Dysanapsis (Mismatch of airway tree caliber to lung size; develops early in life)
- Tobacco smoke
- Nearly 80% of all COPD cases can be attributed to smoking
- 15-20% of 1 pack per day (PPD) smokers and 25% of 2 PPD smokers will develop COPD
- Outdoor and Indoor Pollution
- Biomass fuel
- 50% of COPD deaths in developing countries are from biomass smoke
- 75% are in women
- Biomass fuel
- Occupational exposures
- May account for 15-20% of COPD
- Mining, agriculture, textile, paper, wood, chemical, food processing
- Cadmium fumes can cause emphysema (smelting, batteries)
- Socioeconomic status
- Genetic factors
- Several GWAS (genome-wide association study) have linked genetic loci with COPD but causality remains uncertain
- Best documented in Alpha-I Antitrypsin Deficiency
- SERPINA1 gene mutation
Lung Function Trajectory
- Reduced maximal attained lung function may identify individuals at increased risk
- Incidence of COPD was higher when FEV1 was <80% predicted before age 40 (26% vs 7%; P <0.001)
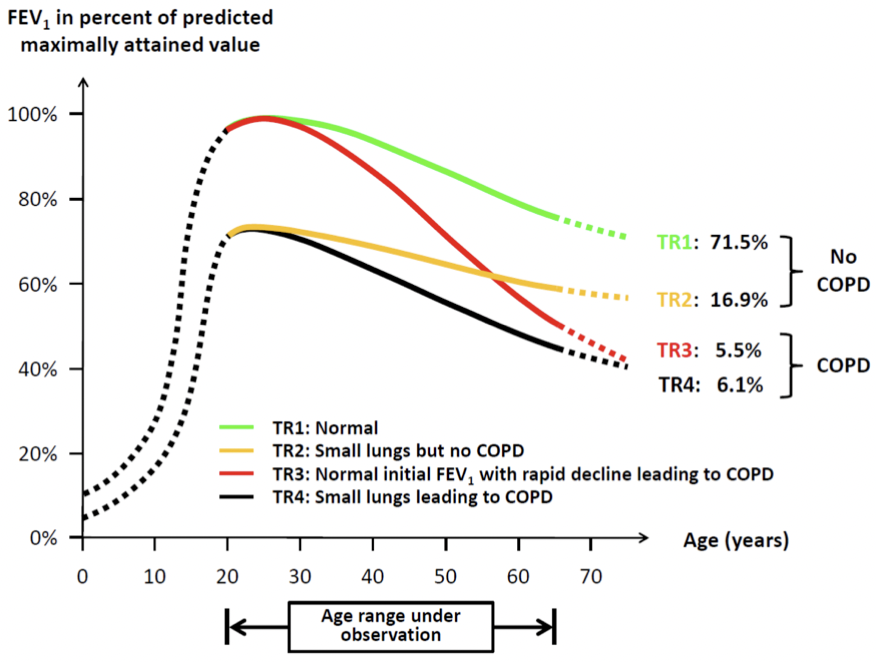
- TR1: Normal
- TR2: Small lungs but no COPD
- TR3: Normal initial FEV₁ with rapid decline leading to COPD
- TR4: Small lungs leading to COPD
Dysanapsis
- Associated with incident COPD in older adults
- Develops early in life
- May contribute to COPD susceptibility later in life
- Three trials -- MESA (N=2531), CanCOLD (N=1272), SPIROMICS (N=2726)
- Incidence and prevalence of COPD was highest in the lowest airway to lung ratio quartiles in MESA and CanCOLD
- SPIROMICS participants with COPD in the highest airway to lung ratio quartiles had faster FEV₁ decline as compared to the lowest airway to lung ratio quartile suggesting two different paths for COPD development
- Can lead to Bronchopulmonary Dysplasia
Risk Factors for AECOPD
- Advanced age
- Severity of airflow limitation
- Chronic mucous hypersecretion
- Productive cough
- Duration of COPD
- Pulmonary Hypertension (Pulmonary Artery {PA}: Ascending Aorta {A} ratio >1)
- Blood eosinophil count >340 cells/μL
- Comorbid Conditions
- GERD
- History of antibiotic use
- COPD-related hospitalization in the previous year
- History of prior exacerbations is the single best predictor, regardless of severity
Etiology of AECOPD
- Causes of exacerbations requiring hospitalization in patients (Papi A, et al. Am J Respir Crit Care Med. 2006;173:1114)
- Non-infectious 21.8%
- Bacteria 29.7%
- Virus 23.4%
- Bacteria & Virus 25%
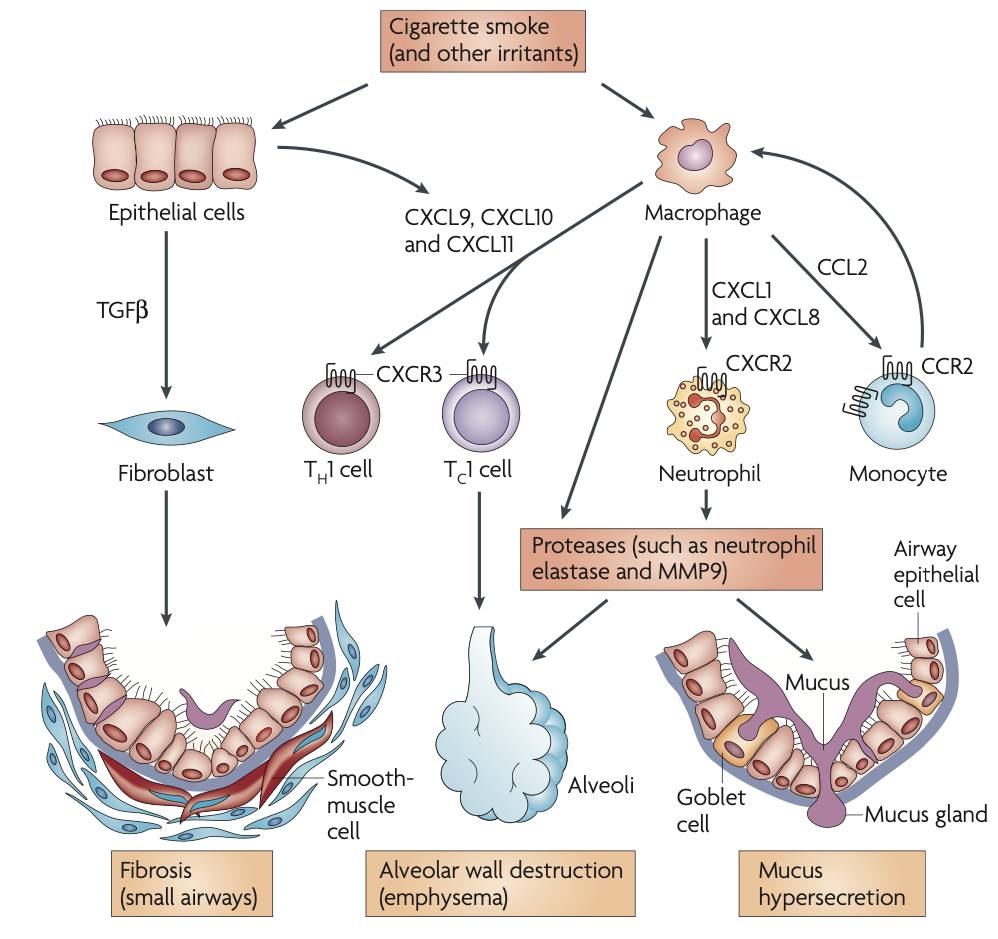
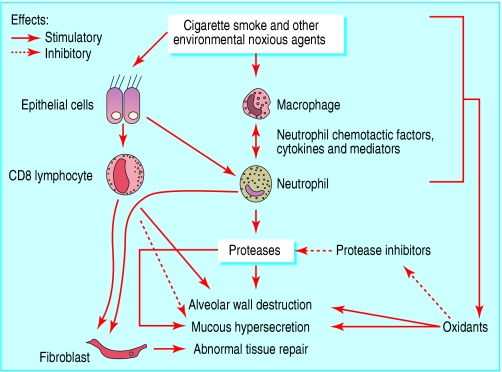
Pathogenesis
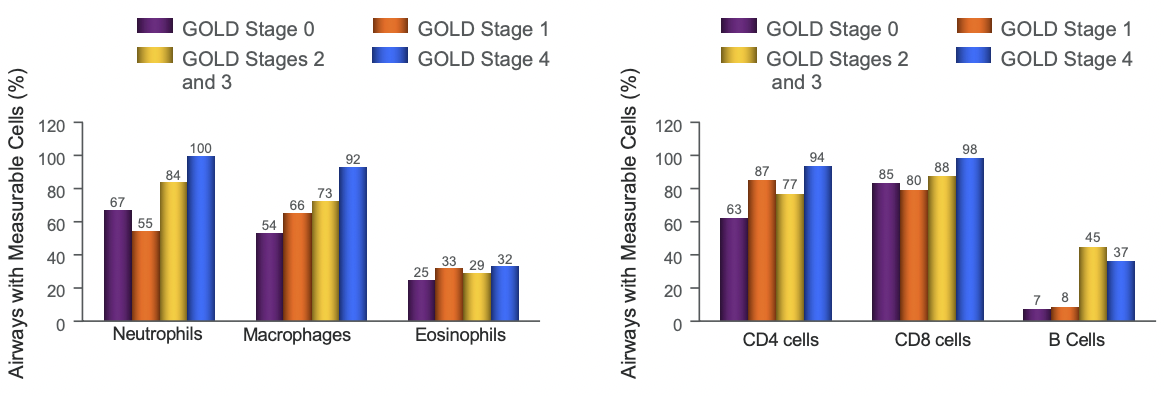
Inflammation in COPD
T Lymphocytes
- Increase in T lymphocytes in the airway wall and lung parenchyma
- organize into lymphoid follicles
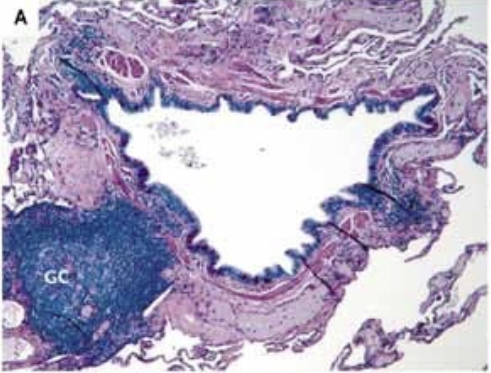
- Collection of bronchial lymphoid tissue with a lymphoid follicle containing a germinal center (GC) surrounded by a rim of darker-staining lymphocytes that extend to the epithelium of both the small airway and alveolar surface
- Shift in the balance of CD4/CD8 T cell ratio in favor of CD8 → direct toxic effects → alveolar wall destruction
- Number of Th1 and Tc1 cells, which produce interferon-γ increase
Neutrophils
- Release proteases
- Increased in the sputum and distal airspaces of smokers
- Further increase occurs in COPD and is related to disease severity
Macrophages
- Produce inflammatory mediators and proteases
- Increased in number in airway, lung parenchyma and in BAL fluid
B Lymphocytes
- increased in peripheral airways and within lymphoid follicles possibly as a response to chronic infection of the airways
Inflammatory Mediators
- Leukotriene B4
- Chemoattractant of neutrophils and T cells
- produced by macrophages, neutrophils, and epithelial cells
- Chemotactic factors (CXC) & Chemokines (IL-8 & growth related oncogene α)
- produced by macrophages and epithelial cells
- Chemoattractant of cells from circulation and amplify pro-inflammatory responses
- TNF-α & IL 1β + IL 6 -- Pro-inflammatory cytokines
- TGF-β -- growth factors
- may cause fibrosis in the airways either directly or through release of another cytokine, connective tissue growth factor
Protease and Antiprotease Imbalance
- Increased production (or activity) of proteases and decreased production (or inactuvation) of antiproteases = imbalance
- Cigarette smoke + inflammation → oxidative stress → primes inflammatory cells → release of proteases and inactivates several antiproteases by oxidation
- Oxidative stress
- leads to inactivation of antiproteases & stimulation of mucous production
- Amplifies inflammation by enhancing transcription factor activation (NFκB) → gene expression of pro-inflammatory mediators
- Oxidative stress
- Main proteases involved
- Neutrophil produced -- serine proteases, elastase, cathepsin G, and protease 3
- Macrophage produced -- cysteine proteases, cathepsins E, A, L, and S
- Matrix metalloproteases -- MMP-8, MMP-9, and MMP-12
- Main antiproteases involved
- α1 antitrypsin
- secretory leukoprotease inhibitor
- tissue inhibitors of metalloproteases
HDAC expression is reduced in COPD
- Histone deacetylase 2 (HDAC2 suppresses inflammatory gene experssion
- Corticosteroids can recruit HDAC and reverse inflammatory process
- HDAC function is impaired by cigarette smoking and oxidative/nitrative stress
- HDAC levls in the lung decrease with increasing COPD severity
- In COPD, there is decreased corticosteroid responsiveness
α-1 Antitrypsin (AAT) Deficiency
- AAT is a protease inhibitor of the proteolytic enzyme elastase
- An imbalance between neutrophil elastase and the elastase inhibitor causes early COPD in smokers (see above)
- COPD occurs later in non-smokers
- Inherited in autosomal co-dominant pattern
- SERPINA1 gene on Chromosome 14
- M allele is normal
- S or Z allele a/w deficiency

- Consider AAT if
- earlier age of onset (<45 years)
- emphysema in a nonsmoker or minimal smoker
- Basal, pan-acinar emphysema
- Unremitting asthma with persistent airflow limitation
- Concurrent liver disease/cirrhosis
- Necrotizing panniculitis
- C-ANCA positive vasculitis
- First-degree relative with emphysema, bronhiectasis, panniculitis
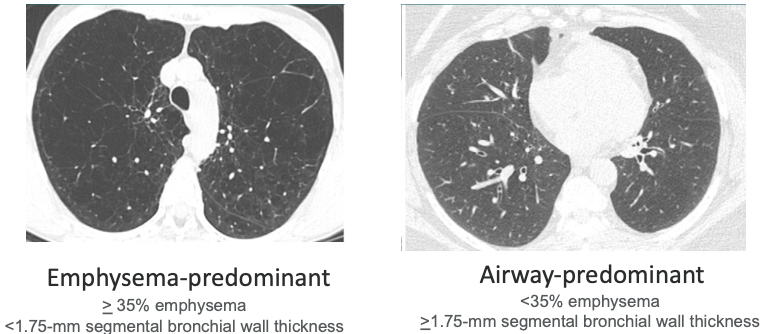
Exacerbation-Prone COPD
- Imaging phenotypes (Han MK, et al. Radiology 2011;261(1):274)
- COPDGene Study
- Greater lung emphysema and airway wall thickness a/w COPD exacerbations, independent of the severity of airflow obstruction
Pathophysiology
Mucus Hypersecretion & Ciliary Dysfunction
Mucus Hypersecretion
- Results in chronic productive cough
- Characteristic of chronic bronchitis but not necessarily a/w airflow obstruction
- Not all patients with COPD have symptomatic mucous hypersecretion
- Hypersecretion is due to
- squamous metaplasia
- increased # of goblet cells
- increased size of bronchial submucosal glands in response to chronic irritation by noxious particles and gases
Ciliary Dysfunction
- Ciliary Dysfunction due to
- squamous metaplasia of epithelial cells
- Abnormal mucociliary escalator
- Difficulty in expectorating
- squamous metaplasia of epithelial cells
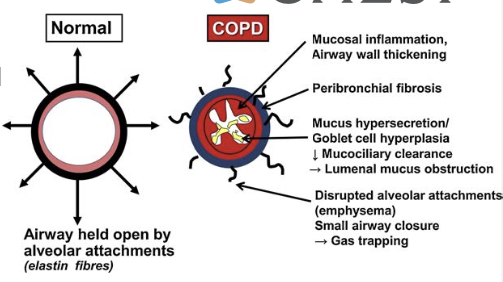
Airflow Limitation & Air Trapping (Hyperinflation)
- Inflammation, fibrosis, and luminal exudates occur in small airways (<2 mm in diameter)
- This is because of inflammation and narrowing (airway remodeling) and inflammatory exudates in the small airways
- Number of small airways is decreased
- smoking-related or may reflect abnormal early-life lung development
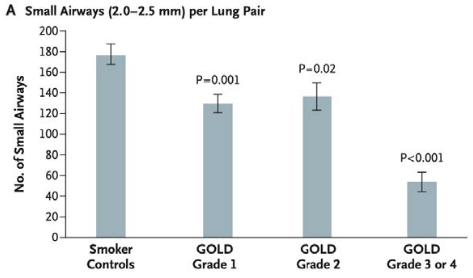
- Hogg J. Lancet 2004; 364: 709–21
- Other factors -- loss of lung elastic recoil (due to destruction of alveolar walls) and destruction of alveolar support (from alveolar attachments)
- Hyperinflation occurs early in the disease and is the main mechanism of exertional dyspnea
- The progressive airway obstruction traps air during expiration resulting in
- hyperinflation at rest
- dynamic hyperinflation during exercise
- Reduces inspiratory capacity → reduction in FRC during exercise → breathlessness & limited exercise capacity
- The progressive airway obstruction traps air during expiration resulting in
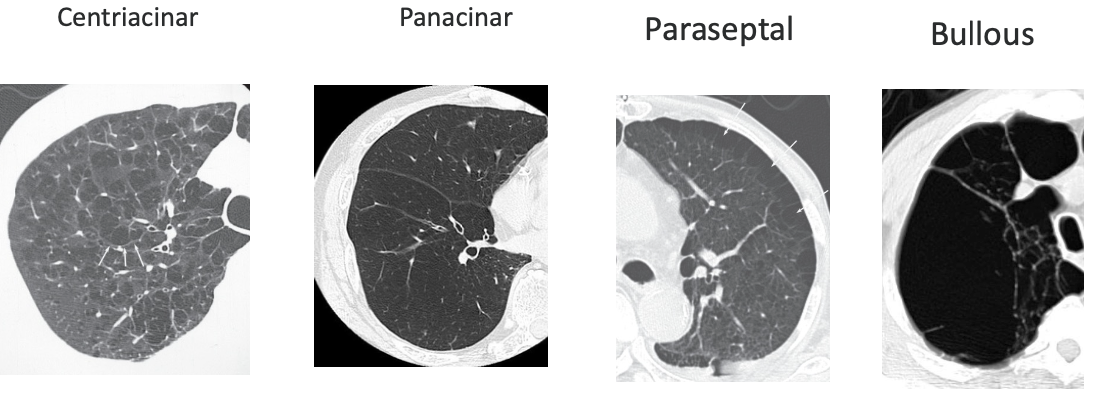
Emphysema & Gas Exchange Abnormalities
- Peribronchiolar destruction of alveolar walls
- Loss of alveolar attachments
- Loss of elastic recoil and airway collapse
- Enlargement of air spaces
Subtypes of Emphysema
Gas Exchange Abnormalities
- Gas exchange abnormalities occur in advanced disease
- Arterial hypoxemia with or without hypercapnia
- Anatomical changes in COPD → abnormal distribution of V/Q ratios → abnormal gas exchange
- Extent of impairment of DLCO per liter of alveolar volume correlates well with the severity of emphysema
- Arterial hypoxemia with or without hypercapnia
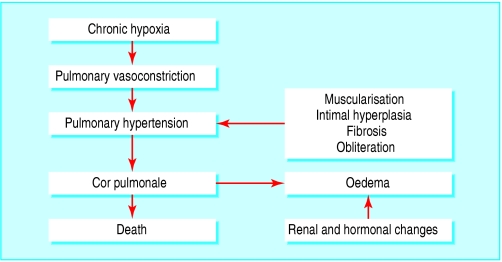
Pulmonary Hypertension
- Develops late in COPD at the time of severe gas exchange abnormalities
- Hypoxia → pulmonary arterial constriction
- Structural change in pulmonary arterioles
- Endothelial dysfunction
- Remodeling of pulmonary arteries (smooth muscle hypertrophy and hyperplasia)
- Destruction of pulmonary capillary bed
- Hypoxia + Structural changes in pulmonary arterioles → persistent pulmonary hypertension & right ventricular hypertrophy or enlargement and dysfunction (cor pulmonale)
Systemic Effects
- Systemic inflammation + skeletal muscle wasting → limited exercise capacity
- worsen prognosis irrespective of degree of airflow obstruction
- Increased risk of CV disease → a/w increase in CRP (see below)
Exacerbation Pathophysiology
- Increased neutrophilic inflammation & increased number of eosinophils (in mild exacerbations)
- Caused by infection, air pollution, and changes in ambient temperature
- Mild exacerbation
- airflow obstruction is unchanged to slightly increased
- Severe exacerbation
- Worsening pulmonary gas exchange due to V/Q mismatch & Respiratory Muscle Fatigue
- Worsening pulmonary gas exchange
- airway inflammation, edema, mucous hypersecretion, and bronchoconstriction → reduce ventilation → hypoxic vasoconstriction of pulmonary arterioles → impairs perfusion
- Respiratory Muscle Fatigue + Alveolar hypoventilation
- Result in hypoxemia, hypercapnia, and respiratory acidosis → severe respiratory failure → death
- Hypoxia + Respiratory acidosis → pulmonary vasoconstriction → increase load on right ventricle + renal and hormonal changes → peripheral edema
History & Physical Exam/Clinical Features
Diagnosis
Consider COPD and Perform Spirometry if:
- Dyspnea -- progressive over time, worse with exercise, persistent
- Chronic Cough -- may be intermittent and dry
- Recurrent Wheezing
- Chronic sputum
- Recurrent respiratory infections
- History of risk factors -- smoke or other noxious, occupational, genetics
- Childhood factors -- prematurity, maternal smoking, low birth weight
- Family history of COPD
- Presence or absence of symptoms (Wheezing, Cough, sputum production or Dyspnea) was not particularly discriminative in diagnosing COPD in the general population
GOLD Grades
- Airflow limitation based on post-bronchodilator FEV₁
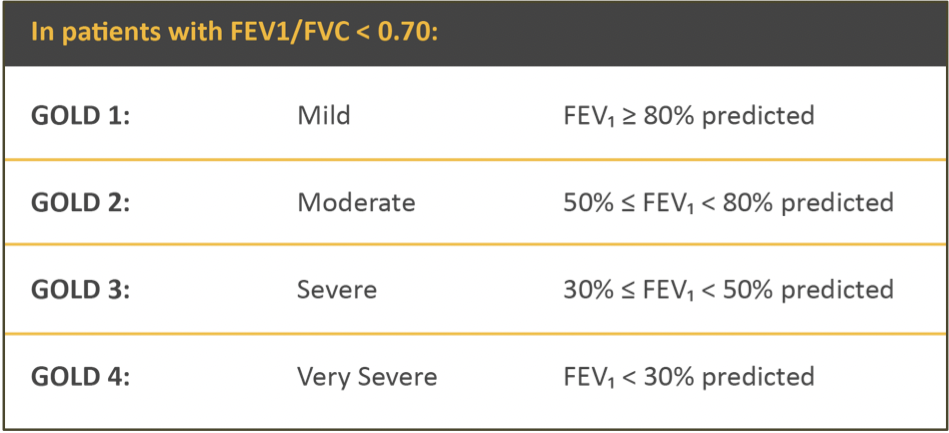
- Assess airflow limitation first then symptoms & risk of exacerbation
- FEV₁ and Mortality from NHANES 1 data
- Increasing mortality with increasing severity of airflow limitation
- Never smokers with moderate-severe COPD were not at increased risk of mortality
Symptom Assessment Tool
mMRC Dyspnea Scale
COPD Assessment Tool (CAT)
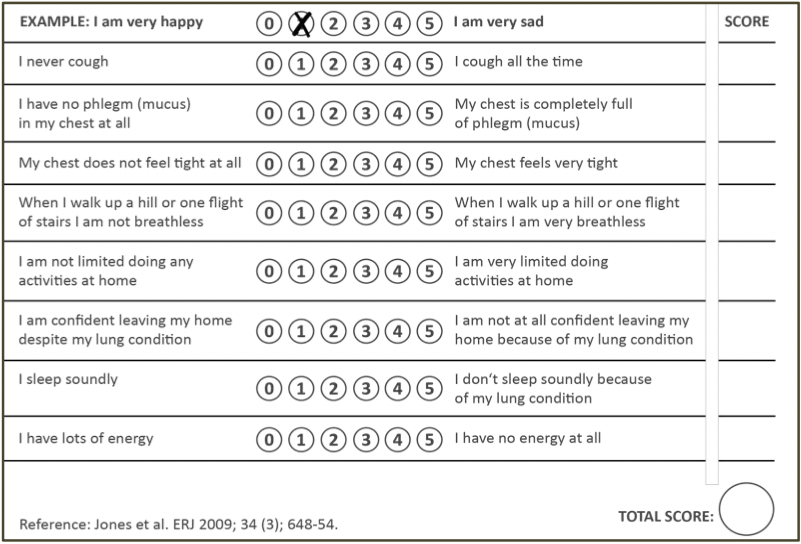
- 8 items; scale 1-5
- Scores 0 - 40
- <10 - Low impact
- 10-20 - Medium impact
- 21-30 - High impact
-
30 - Very high impact
- Fixes limitation with predicting risk of acute exacerbation based on FEV₁
- Assess symptoms plus consequences (activity limitation, sleep, fatigue, self-efficacy)
Additional Investigations to Consider
- α-1 antitrypsin (AAT) screening
- WHO recommends screening all patients with COPD at least once
- Imaging
- Lung volumes and diffusing capacity
- Oximetry and blood gas measurement
- Exercise testing
- Composite scores (such as BODE index for COPD survival)
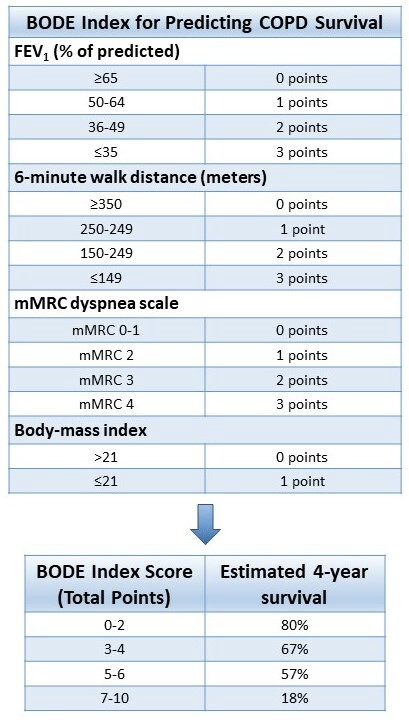
- B- BMI, O- degree of airflow Obstruction (FEV₁%), D-Dyspnea (mMRC), E-Exercise capacity index (6MWT)
- BODE is a better predictor of risk of death from any cause and from respiratory causes than is the FEV₁ alone
- FEV₁ does not adequately reflect all the systemic manifestations of the disease
- FEV₁ correlates weakly with degree of dyspnea and change in FEV₁ does not reflect the rate of decline in patient's health
- Degree of dyspnea and health-status scores are more accurate predictors of risk of death than is the FEV₁.
- Biomarkers -- need further study
α1 Anti-Trypsin Deficiency
Diagnosis of AECOPD
Management
Management of Stable COPD
- Goals of COPD Management
- Reduce Symptoms
- relieve symptoms of dyspnea
- improve exercise tolerance
- improve health status
- Reduce Risk
- prevent disease progression
- prevent and treat exacerbations
- reduce mortality
- Reduce Symptoms
Pharmacologic Therapy
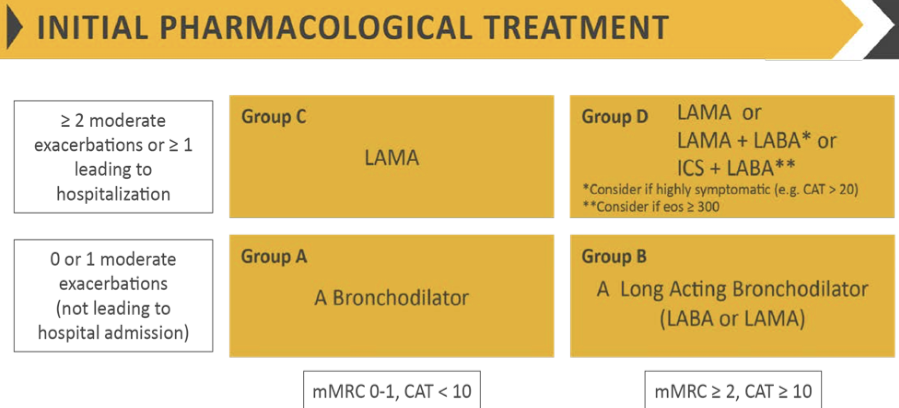
- Symptomatic patients with mild to moderate COPD (post-bronchodilator FEV₁ ≥50% predicted), there is significant improvement of annual decline in FEV₁ after bronchodilator use, accompanied by a significant increase in the time to first AECOPD, but no improvement in mortality.
- Improvement in prebronchodilator FEV₁ and post, mMRC and CAT scores
- SABA and SAMA improve FEV₁ and symptoms
- SABA + SAMA > SABA or SAMA
- LAMA and LABA improve lung function (FEV₁), dyspnea (symptoms), health status and exacerbations
- LAMA -- greater effect on exacerbation and hospitalization reduction
- LAMA -- improve effectiveness of pulmonary rehabilitation
- LAMA + LABA > LAMA or LABA
- Inhaled Steroids
- ICS + LABA -- more effective than ICS or LABA in improving lung function, health status and exacerbations
- Triple inhaled therapy (ICS/LABA/LAMA)
- improves lung function, symptoms, health status, exacerbations, mortality vs
- ICS/LABA or LABA/LAMA or LAMA
- improves lung function, symptoms, health status, exacerbations, mortality vs
- Regular treatment with ICS increases risk of pneumonia, especially in those with severe disease
- Factors to consider when initiating ICS treatment
- Data
- ISOLDE Study (Burge PS, et al. BMJ. 2000;320:1297)
- ICS (Fluticasone) decreases COPD exacerbation risk by 25%
- UPLIFT Study (Tashkin DP, et al. N Engl J Med. 2008;359:1543)
- LAMA (Tiotropium) decreases COPD exacerbation risk by 14%
- TORCH Study (Calverley PM, et al. N Engl J Med. 2007;356:775)
- LABA + ICS (Salmeterol + Fluticasone) decreases COPD exacerbation more than ICS or LABA alone
- POET-COPD Study (Vodelmeier et al. NEJM 2011;364:1093)
- LAMA (Tiotropium) decreases exacerbations more than LABA (Salmeterol)
- FLAME Study (Wedzicha et al. NEJM 2016; 374: 2222)
- LAMA + LABA (Glycopyrronium + Indacaterol) decreases exacerbations more than ICS + LABA (Fluticasone + Salmeterol)
- Effect independent of baseline blood eosinophil count
- Higher incidence of pneumonia in ICS group
- ETHOS Study (Rabe et al. NEJM 2020; 383:35) -- looked at exacerbation rate
- Large study - 8509 patients with moderate to very severe COPD
- ≥1 moderate to severe exacerbation in prior year if FEV₁ <50%
- ≥2 moderate or ≥1 severe exacerbation if FEV₁ 50-65%
- ICS used was Budesonide 160 and 320
- Triple therapy with Budesonide 160 had lowest rate of moderate to severe exacerbation compared to triple therapy with Budesonide 320 and ICS + LABA and LAMA + LABA (highest rate) -- surprising that LAMA + LABA had a higher exacerbation rate than ICS + LABA when FLAME study said opposite (potentially a result of the individual drugs used?)
- Large study - 8509 patients with moderate to very severe COPD
- IMPACT Study (Lipson et al. AJRCCM 2020) -- looked at mortality
- Large study - 10355 patients with symptomatic COPD; FEV₁ <50% and at least 1 moderate to severe exacerbation in the prior year; FEV₁ 50 - <80% and at least 1 severe or 2 moderate exacerbations (a little higher cut off than ETHOS study)
- LAMA + LABA highest all-cause mortality
- ICS + LABA & triple therapy had similar all-cause mortality at the end of 52 weeks
- Macrolide Therapy in COPD & Exacerbations
- Long-term macrolide therapy reduces exacerbations (Seemungal et al. AJRCCM 2008)
- medium time to 1st exacerbation 271 days macrolide; 89 days for placebo
- Azithromycin therapy (Albert et al NEJM 2011; 365:689)
- medium time to exacerbation 266 days azithromycin; 174 days placebo
- Long-term macrolide therapy reduces exacerbations (Seemungal et al. AJRCCM 2008)
- Roflumilast & Exacerbations (Martinez et al Lancet 2015;385:857)
- All patients had baseline LABA/ICS use
- 13% reduction in exacerbations
- ISOLDE Study (Burge PS, et al. BMJ. 2000;320:1297)
Non-Pharmacologic Therapy
- Smoking cessation
- Effect on Lung Function (The Lung Health Study at 11 years Anthonisen et al. Am J Respir Crit Care Med. 2002;166:675)
- Men who quit had an FEV₁ rate of decline of 30 mL/year
- Men who continued to smoke had an FEV₁ rate of decline of 66 mL/year
- Effect on Lung Function (The Lung Health Study at 14 years Anthonisen NR, et al. Ann Intern Med. 2005;142:233)
- Sustained quitters had the least deaths per 1000 person-years followed by intermittent quitters and highest deaths per 1000 person-years for continued smokers
- Effect on Lung Function (The Lung Health Study at 11 years Anthonisen et al. Am J Respir Crit Care Med. 2002;166:675)
- Immunization
- Influenza vacciantion reduces serious illness and death in COPD patients
- PPSV 23 has been shown to reduce the incidence of CAP in
- COPD patients aged <65 years + FEV₁ <40% predicted
- Those with comorbidities
- PCV 13 demonstrated significant efficacy in reducing bacteremia and serious invasive pneumococcal disease in adults ≥65 years
- No longer recommended by CDC unless specific risk factors
- Tdap vaccination recommended in adults with COPD who were not vaccinated in adolescence to protect against pertussis
- Pulmonary rehabilitation (Alison et al. Respirology 2017;22(4):800 McCarthy et al. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No.: CD003793)
- Significantly improves exercise capacity and health status (QOL/anxiety/depression/dyspnea)
- Reduces frequency of exacerbations
- Reduces the number of readmissions in the year following initiation
- Reduction in mortality
- Optimum benefit from programs 6-8 weeks' duration
- does not improve pulmonary function tests or oxygenation
- Oxygen therapy
- Long-term oxygen therapy (LTOT) = >15 hours/day
- Severe chronic resting arterial hypoxemia (PaO₂ ≤55 mm Hg)
- Long-term oxygen therapy improved survival vs nocturnal-only or no oxygen
- Moderate chronic resting hypoxemia (PaO₂ 56-59 mm Hg or SpO₂ 88-90%) + cor pulmonale or polycythemia
- Long-term oxygen therapy improved survival
- Moderate chronic resting hypoxemia (SpO₂ 89-93%)
- Long-term oxygen therapy does not lengthen time to death or time to first hospitalization or provide sustained benefit in health status, lung function and 6MWT distance
- Exercise-Induced Hypoxemia (SpO₂ during 6MWT ≥80% for ≥5 minutes and <90% for ≥10 seconds)
- Long-term oxygen therapy does not lengthen time to death or time to first hospitalization or provide sustained benefit in health status, lung function and 6MWT distance
- Use of supplemental oxygen during exercise, however, increases exercise performance
- SpO₂ 80 to 88% during exercise (Moderate Exercise Hypoxemia), → O₂ during exercise did not improve long term outcomes of hospitalization, QOL, dyspnea
- Supplemental oxygen may remove the stimulus to hypoxia-compensating mechanisms such as those seen in residents of high-altitude
- Long-term oxygen therapy does not lengthen time to death or time to first hospitalization or provide sustained benefit in health status, lung function and 6MWT distance
- Indications in Stable COPD
- Resting Hypoxemia
- PaO₂ ≤55 mm Hg or SaO₂ ≤88%
- PaO₂ ≤59 mm Hg or SaO₂ ≤ 89% +
- EKG evidence of cor pulmonale
- Hematocrit > 55
- Clinical evidence of right heart failure
- Exercise-Induced Hypoxemia
- PaO₂ ≤55 mm Hg during exercise (Severe Exercise Hypoxemia) → O₂ prescribed for use DURING exercise
- Unknown if this has any effect on long-term outcome benefits
- Use during exercise did not translate to improvement/benefit in dyspnea or ADLs when not using oxygen
- PaO₂ ≤55 mm Hg during exercise (Severe Exercise Hypoxemia) → O₂ prescribed for use DURING exercise
- Sleep-Induced Hypoxemia
- PaO₂ ≤55 mm Hg during sleep → O₂ prescribed
- Also need to evaluate with PSG for underlying sleep-disordered breathing
- Resting Hypoxemia
- Supplemental oxygen sometimes provides symptomatic dyspnea relief by stimulating a decrease in minute ventilation → may improve activity but does not improve survival
- COPD + DOE can be from exertional hypoxemia but also from mechanical loading of the respiratory system, deconditioning and concomitant cardiac disease → pulmonary rehabilitation
- Non-invasive positive pressure ventilation
- In stable hypercapnic COPD improves survival (Kohnlein et al. Lancet Respir Med 2014;2:698)
- Criteria: GOLD stage IV COPD + PaCO₂ ≥ 52 mm Hg & pH > 7.35
- Long-term NPPV targeted to reduce hypercapnia
- 11% reduction in mortality at 1 year
- In patients admitted for AECOPD requiring NIV and who remain hypoxemic and hypercarbic (PaCO₂ ≥52 mm Hg) 2 weeks following discharge, the addition of goal-directed nocturnal NIV to continous O₂ has been shown to significantly prolong time to hospital readmission and death within 12 months + improved health-related QOL and reduction in AECOPD frequency
- AECOPD + NIV = poor prognosis w/ median time to readmission or death less than a year
- benefit from supplemental oxygen
- use NIV for at least 6 hours at night → reduce nocturnal hypoventilation → reduction in daytime PaCO₂ by 6-8 mm Hg
- AECOPD + NIV = poor prognosis w/ median time to readmission or death less than a year
-
Interventional & Surgical Therapy in Stable COPD
- Surgical Lung Volume Reduction (NETT research group NEJM 2003;348:2059 Naunheim et al. Ann Thorac Surg 2006; 82:431)
- Upper lobe emphysema a/w improvement in exercise capacity & QOL
- Upper lobe emphysema + low baseline exercise capacity a/w improvement in survival
- Bronchoscopic Lung Volume Reduction w/ Endobronchial Valves (EBV) (Criner et al. AJRCCM 2018;198(9):1151 Kemp et al. AJRCCM 2017;196(12):1535 Majid et al. Respiration 2020;99(1):62)
- Criteria
- hyperinflation due to severe emphysema
- symptomatic despite optimal medical therapy and pulmonary rehabilitation
- EBV placed in lung region with most emphysematous destruction and no collateral ventilation
- Zephyr duckbill shaped and Spiration umbrella-shaped EBV approved in USA
- Efficacy -- improved FEV₁, dyspnea, 6MWD, QOL, RV
- A/E -- significant risk of pneumothorax (25-30%)
- EBV does not preclude subsequent LVRS or transplant
| Reduce Exacerbations | Reduce Mortality |
| --------------------------------- | ---------------------------- |
| LABA, LAMA, LABA + LAMA | LABA + LAMA + ICS |
| LABA + ICS, LABA + LAMA + ICS | Smoking Cessation |
| Roflumilast | LVRS |
| Chronic macrolide | NIPPV |
| Smoking Cessation | Pulmonary Rehabilitation |
| Vaccines | |
| Pulmonary Rehabilitation | |
| LVRS | |
| N-acetylcysteine | |
| Vitamin D | |
- Criteria
- Surgical Lung Volume Reduction (NETT research group NEJM 2003;348:2059 Naunheim et al. Ann Thorac Surg 2006; 82:431)
-
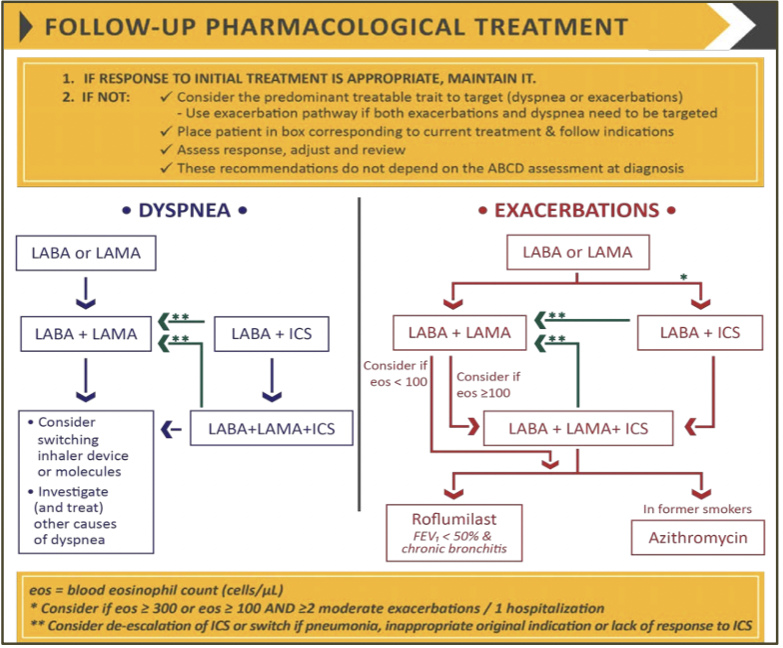
Management of Acute Exacerbations
- SABA with or without SAMA are recommended as initial bronchodilators
- Systemic corticosteroids improve FEV₁, oxygenation and shorten recovery time and duration of hospitalization
- Duration of therapy no more than 5-7 days
- Antibiotics if signs of bacterial infection
- shorten recovery time, reduce risk of early relapse and treatment failure, reduce hospital duration
- Methylxanthines not recommended
- NIV for AECOPD
- If no contraindication, NIV in patients with acute respiratory failure 2/2 AECOPD
- improves gas exchange
- reduces work of breathing
- reduces need for intubation
- reduces hospital duration and hospital re-admission
- improves survival
- If no contraindication, NIV in patients with acute respiratory failure 2/2 AECOPD
Complication & Prognosis
- Diagnosed COPD
- Asymptomatic individuals with FEV₁ <80% (moderate or more severe obstruction) experience significant adverse events similar to symptomatic COPD
- Undiagnosed COPD
- Symptomatic individuals with undiagnosed COPD were found to have an increased risk of exacerbations, pneumonia and death
- Asymptomatic individuals with undiagnosed COPD had an increased risk of exacerbations and pneumonia
Comorbidities ^4e379d
- Heart failure - prevalence 20-70%
- CAD - increased risk of MI for 30 days after acute exacerbation of chronic bronchitis (AECB)
- COPD is an independent risk factor for CV disease
- increased the odds of having CV disease by 2.7
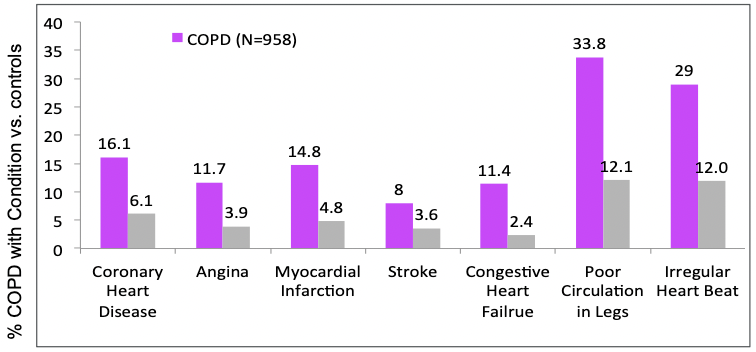
- Finkelstein J, et al. Int J Chron Obstruct Pulmon Dis. 2009;4:337
- Effects of airflow obstruction and systemic inflammation are additive
- Increase in cardiac infarction injury score as airflow obstruction gets worse
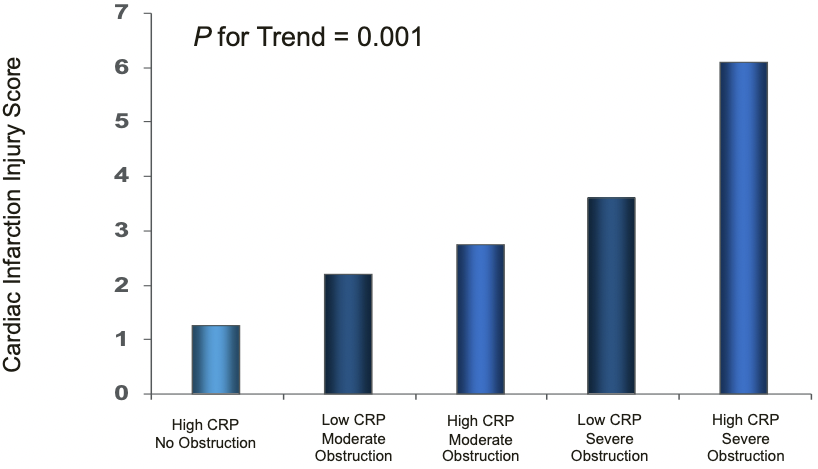 (Sin
(Sin - DD & Man SF. Circulation 2003; 107: 1514
- COPD is an independent risk factor for CV disease
- PVD - higher prevalence in COPD (8.8% vs 1.8%)
- Osteoporosis - associated with emphysema, low BMI, low fat-free mass
- Severity of airflow obstruction predicts osteoporosis
- Worsening severity of airflow obstruction -> increasing percentage of osteoporosis
- More prominent in women than men
- Metabolic syndrome - prevalence >30%
- GERD - independent risk factor for exacerbations
- OSA overlap with COPD a/w worse prognosis
Impact on Mortality
- Estimated age-adjusted mortality a/w selected comorbid conditions
- Holguin et al. CHEST 2005;128(4):2005 - National Hospital Discharge Survey 1979-2001; 47 million discharges
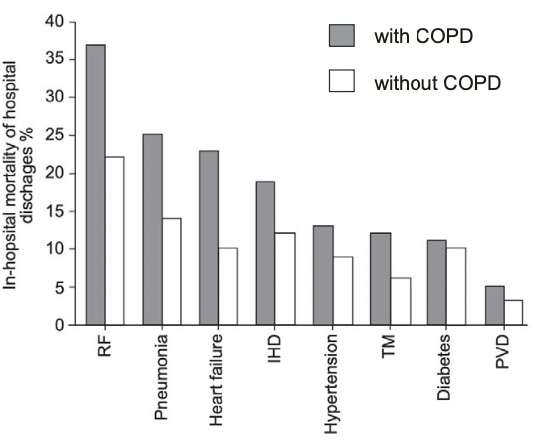
- Those with COPD and comorbid condition had higher in-hospital mortality from the percentage of people that were discharged
Frequent Exacerbations & Decline in Lung Function
- More exacerbations = faster decline in lung function
- Donaldson GC, et al. Thorax. 2002;57:847
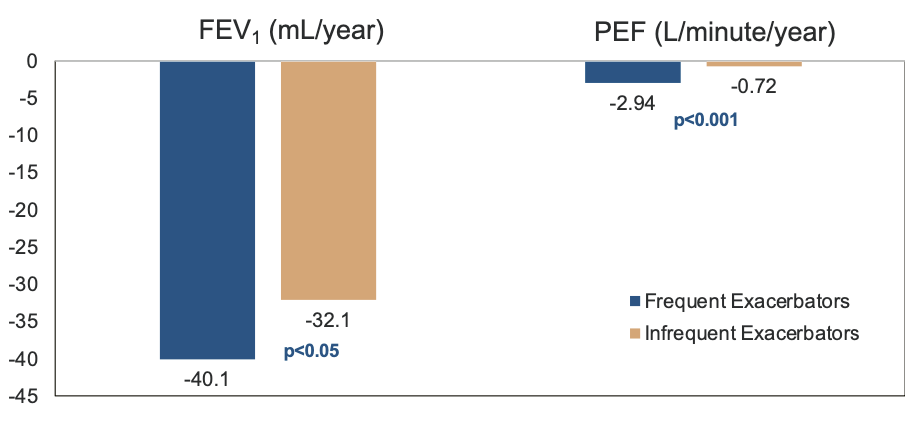
- PEFR recovery after exacerbation (Seemungal TA, et al. Am J Respir Crit Care Med. 2000;161:1608)
- Recovery of PEFR to baseline values was complete in only 75% of exacerbations at 35 day
- In 7% of exacerbations at 91 days PEFR recovery had not occurred
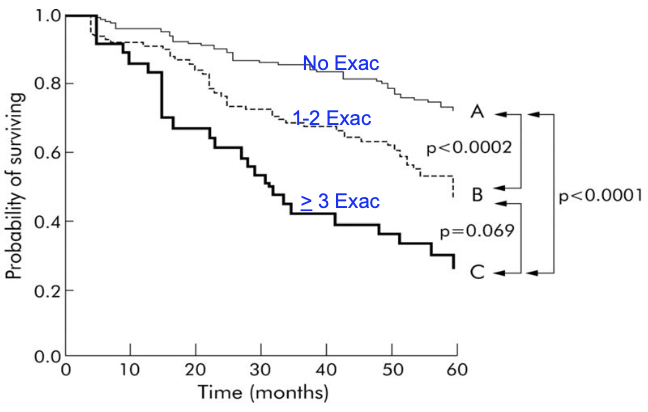
COPD Exacerbations Impact Survival
- Severe COPD Exacerbations
- Soler-Cataluña JJ et al Thorax 2005;64:925
- 304 male patients, hospitalized for AECOPD followed for 5 years
- Risk of death 4.3 times greater in frequent exacerbators
- In general exacerbations are a/w increased mortality
- In patients hospitalized with AECOPD
- 14% mortality in 3 months
- 28% mortality in 1 year
- If AECOPD w/ PaCO₂ > 50 mm Hg, a/w
- 33% mortality in 6 months
- 43% mortality in 12 months
- Roberts et al. Thorax 2002;57(2):137 Connors et al. AJRCCM 1996; 154(4):959 Slenter et al. Respiration 2013;85(1):15 Almagro et al. Respiration 2012;84(1):36
- In patients hospitalized with AECOPD
Backlinks
- 01.1. Obstructive Lung Diseases
- Chronic Obstructive Pulmonary Disease
- Acute Respiratory Distress Syndrome
- Hypercapnic Respiratory Failure
- 10.3. Airway Diseases in Critical Care